-
Alligator Clip Adapter $230.65 / CaseAdd to cart
Alligator Clip Adapter OEM Part Number Compatibility Cross References:
- Manufacturer: Danlee Medical
- OEM Part #: AD125
- Manufacturer: GE Healthcare > Marquette
- OEM Part #: 9490-106
- Manufacturer: Philips
- OEM Part #: 989803129231
- Manufacturer: Sage
- OEM Part #: AL10-3BF
Technical Specifications:
- Category: EKG
- Certifications: FDA, CE, ISO10993-1, 5, 10:2003E, TUV, RoHS Compliant
- Connector Distal: 3mm
- Connector Proximal: Needle Alligator Clip
- Latex-free: Yes
- Lead Color Coding: AHA & IEC
- Packaging Type: Bag
- Packaging Unit: 10
- Patient Size: Adult/Pediatric
- Sterile: No
- Warranty: 6 months
In Stock
- Manufacturer: Danlee Medical
-
Banana Alligator Clip Adapters $230.65 / CaseAdd to cart
Banana Alligator Clip Adapters OEM Part Number Compatibility Cross References:
- Manufacturer: Danlee Medical
- OEM Part #: ADAK10
- Manufacturer: GE Healthcare > Marquette
- OEM Part #: 9490-105, 9490-111
- Manufacturer: Sage
- OEM Part #: AL10-4BF
- Manufacturer: Welch Allyn
- OEM Part #: 58581-0000
Technical Specifications:
- Category: EKG
- Certifications: FDA, CE, ISO10993-1, 5, 10:2003E, TUV, RoHS Compliant
- Connector Distal: 4mm
- Connector Proximal: Banana Alligator Clip
- Latex-free: Yes
- Lead Color Coding: AHA & IEC
- Packaging Type: Bag
- Packaging Unit: 10
- Patient Size: Adult/Pediatric
- Sterile: No
- Warranty: 6 months
In Stock
- Manufacturer: Danlee Medical
-
Disposable Non-invasive EEG Sensor $495.00Select options
The Disposable Non-invasive EEG Sensor is designed for high-performance monitoring of brain activity, compatible with various anesthesia depth monitoring technology modules. It is specifically tailored to meet the needs of hospitals and medical institutions, offering compatibility with numerous brands and models of equipment. The sensor provides accurate and reliable brain monitoring without the need for excessive preparation or invasive procedures, ensuring patient comfort and ease of use for healthcare professionals.
In Stock
-
ENFit Transition Connector $105.91 / CaseAdd to cart
ENFit Transition Connector is used as a connector for ENFit syringe or ENFit feeding set. It also connects to a non ENFit feeding tube. Enteral Feeding tube is used to supply food in liquid form to the patients who cannot get enough nutrition through eating or drinking. Sometimes the tubes are short to reach the patient. In this case, Medline ENFit Transition Connector is of great help to maintain contact with the patient.
*purchase of this item is restricted to health care professional, government agencies, hospitals or patients with a valid prescription
** Min order of 10 cases
*** Free shipping doe not apply to this item
In Stock
-
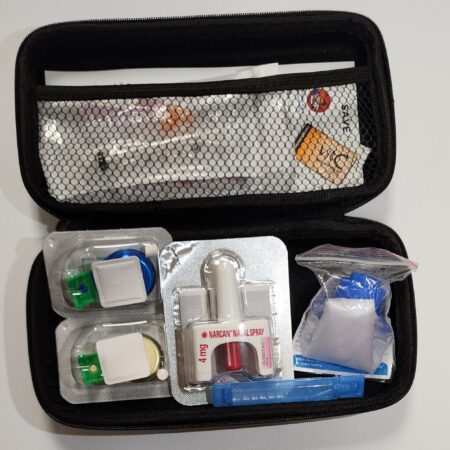
Harm Reduction Kit With Naloxone $99.95 / EachAdd to cart
Comprehensive Harm Reduction Kit with Naloxone has been thoughtfully assembled to provide individuals and communities with essential tools to address potential overdose situations and promote harm reduction practices. This carefully curated kit contains a range of components designed to enhance safety and empower those who may find themselves in critical moments.
Content
- Sterile Vitamin C
- Colour-Coded Sterile Cooker X2
- Optional Naloxone Injection Or Spray
- 25G Single Stroke Saftey Needle 3ML X2
- Sterile Water 3ML
- CPR Barrier Mask
- Nitrile Gloves X2
- Quick guide In French & English
In Stock
-
PHILIPS Fetal Monitoring Chart Paper For M1913A $185.00 / CaseAdd to cart
PHILIPS Fetal Monitoring Chart Paper For M1913A
MECP has Fetal Monitoring Chart Paper for model M1913A available and ready to ship. Designed with easy-to-read grids, extended timelines at one-minute intervals, and consecutively numbered pages, our chart paper ensures clear readings and easy documentation (M1913A).
40 pad/case
In Stock
-
PHILIPS Replacement Thermal Paper M1708A & M2481A $406.20 / CaseAdd to cart
Replacement thermal paper for Philips Cardiograph TC70/50/30 and Stress systems ST80i is in stock. Due to an extended back order from Philips until the end of the year, we offer compatible replacements for the unavailable Philips paper (p/n M1708A and M2481A).
10 Reams per case, 200 sheets per ream
In Stock
-
Semaglutide API $1,000.00Add to cart
Semaglutide API Product Description:
COA Available Product with NDC CodeAvailable in the US and Canada.
Semaglutide API is indicated for improving glycemic control in adults with type 2 diabetes mellitus, in conjunction with diet and exercise. The approved therapeutic doses are 0.5 mg and 1 mg. Type 2 diabetes is a chronic metabolic disorder characterized by high blood sugar, insulin resistance, and insufficient insulin production. The onset of this condition is linked to the inability of beta cells to respond to increased plasma glucose, predominantly caused by lifestyle factors such as overweight and obesity. The key feature of type 2 diabetes is insulin resistance, which reduces insulin’s effectiveness at normal concentrations. Insulin secretion is stimulated by incretins like glucagon-like peptide 1 (GLP-1), which also delays gastric emptying and induces satiety.
Mode of Action:
Semaglutide API enhances glucose control through various mechanisms, including increased insulin secretion, slower gastric emptying, and reduced postprandial glucagon and food intake. The drug mimics the activity of GLP-1, a key hormone in glucose management, thanks to structural modifications that improve its stability against degradation by enzymes like dipeptidyl peptidase-4 (DPP-4). These modifications also promote specific binding to plasma albumin and prevent incorrect binding of fatty acids, resulting in an extended half-life and improved patient compliance.Pharmacodynamics:
Clinical trials show that semaglutide significantly reduces glycated hemoglobin (HbA1c) compared to other medications, including sitagliptin, exenatide, and insulin glargine U100. HbA1c is a standard measure of high glucose levels, reflecting long-term blood sugar control. Additionally, semaglutide has been shown to reduce body weight, lower fasting and postprandial glucose levels, and decrease fasting triglycerides and VLDL cholesterol.Metabolism:
Semaglutide is slowly but extensively metabolized before excretion, with 83% of the administered dose found unchanged in plasma. Six different metabolites have been identified, with the major metabolite accounting for 7.7% of the dose. The drug is primarily degraded by enzymes such as DPP-4 and neural endopeptidase (NEP), which inactivate semaglutide and hydrolyze its peptide bonds.Toxicity:
In preclinical studies, mild c-cell hyperplasia and other effects, including liver necrosis and centrilobular hypertrophy, were observed at doses significantly higher than those used clinically. High doses also resulted in ECG abnormalities and tissue vacuolation.IUPAC:
17-{[(1R)-3-[(2-{2-[({2-[2-({[(5S)-5-[(2S)-2-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S,3R)-2-[(2S)-2-[(2S,3R)-2-{2-[(2S)-2-{2-[(2S)-2-amino-3-(1H-imidazol-4-yl)propanamido]-2-methylpropanamido}-4-carboxybutanamido]acetamido}-3-hydroxybutanamido]-3-phenylpropanamido]-3-hydroxybutanamido]-3-hydroxypropanamido]-3-carboxypropanamido]-3-methylbutanamido]-3-hydroxypropanamido]-3-hydroxypropanamido]-3-(4-hydroxyphenyl)propanamido]-4-methylpentanamido]-4-carboxybutanamido]acetamido}-4-carbamoylbutanamido]propanamido]propanamido]-5-{[(1S)-1-{[(1S)-1-{[(1S,2S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-4-carbamimidamido-1-[({[(1S)-4-carbamimidamido-1-[(carboxymethyl)carbamoyl]butyl]carbamoyl}methyl)carbamoyl]butyl]carbamoyl}-2-methylpropyl]carbamoyl}-3-methylbutyl]carbamoyl}-2-(1H-indol-3-yl)ethyl]carbamoyl}ethyl]carbamoyl}-2-methylbutyl]carbamoyl}-2-phenylethyl]carbamoyl}-3-carboxypropyl]carbamoyl}pentyl]carbamoyl}methoxy)ethoxy]ethyl}carbamoyl)methoxy]ethoxy}ethyl)carbamoyl]-1-carboxypropyl]carbamoyl}heptadecanoic acidDrugBank:
DB13928Formula:
C187-H291-N45-O59Molecular Mass:
Average: 4113.641
Monoisotopic: 4111.11537713Synonyms:
Semaglutide / Semaglutide API
SMILES:
CC[C@H](C)[C@H](NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCNC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1=CC=C(O)C=C1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)CC1=CNC=N1)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O
InChi:
InChI=1S/C187H291N45O59/c1-18-105(10)154(180(282)208-108(13)159(261)216-133(86-114-89-200-119-50-40-39-49-117(114)119)170(272)218-129(82-102(4)5)171(273)228-152(103(6)7)178(280)215-121(53-44-72-199-186(192)193)162(264)201-91-141(242)209-120(52-43-71-198-185(190)191)161(263)204-94-151(257)258)230-172(274)131(83-111-45-33-31-34-46-111)219-167(269)126(64-69-149(253)254)214-166(268)122(51-41-42-70-195-144(245)98-290-79-78-289-76-74-197-145(246)99-291-80-77-288-75-73-196-139(240)66-61-127(183(285)286)211-140(241)54-37-29-27-25-23-21-19-20-22-24-26-28-30-38-55-146(247)248)212-158(260)107(12)206-157(259)106(11)207-165(267)125(60-65-138(189)239)210-142(243)92-202-163(265)123(62-67-147(249)250)213-168(270)128(81-101(2)3)217-169(271)130(85-113-56-58-116(238)59-57-113)220-175(277)135(95-233)223-177(279)137(97-235)224-179(281)153(104(8)9)229-174(276)134(88-150(255)256)221-176(278)136(96-234)225-182(284)156(110(15)237)231-173(275)132(84-112-47-35-32-36-48-112)222-181(283)155(109(14)236)227-143(244)93-203-164(266)124(63-68-148(251)252)226-184(287)187(16,17)232-160(262)118(188)87-115-90-194-100-205-115/h31-36,39-40,45-50,56-59,89-90,100-110,118,120-137,152-156,200,233-238H,18-30,37-38,41-44,51-55,60-88,91-99,188H2,1-17H3,(H2,189,239)(H,194,205)(H,195,245)(H,196,240)(H,197,246)(H,201,264)(H,202,265)(H,203,266)(H,204,263)(H,206,259)(H,207,267)(H,208,282)(H,209,242)(H,210,243)(H,211,241)(H,212,260)(H,213,270)(H,214,268)(H,215,280)(H,216,261)(H,217,271)(H,218,272)(H,219,269)(H,220,277)(H,221,278)(H,222,283)(H,223,279)(H,224,281)(H,225,284)(H,226,287)(H,227,244)(H,228,273)(H,229,276)(H,230,274)(H,231,275)(H,232,262)(H,247,248)(H,249,250)(H,251,252)(H,253,254)(H,255,256)(H,257,258)(H,285,286)(H4,190,191,198)(H4,192,193,199)/t105-,106-,107-,108-,109+,110+,118-,120-,121-,122-,123-,124-,125-,126-,127+,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,152-,153-,154-,155-,156-/m0/s1Purchase of this product is restricted to licensed companies and labs.
In Stock
-
Specimen Bags $122.52 / CaseAdd to cart
Designed for use in hospitals and medical labs, this zipper bag ensures the safe transfer of specimens, paperwork, and sample cups. The main compartment securely holds specimens, preventing spills, while the outer pouch keeps paperwork separate and protected.
- 2ml thickness
In Stock
-
Welch Allyn Compatible Direct-Connect EKG Cable From: $755.20Select options
Welch Allyn Direct-Connect EKG Cable, compatible with PCR 100 Resting ECG and SE-PRO-600 Cardioperfect Pro Recorder, features a 6 ft total length, latex-free TPU jacket, and FDA, CE, ISO10993-1, 5, 10:2003E, TUV, and RoHS certifications. Designed for both adult and pediatric patients, it includes 10 AHA color-coded leads, Direct-Connect EKG Cable ensuring reliable and safe performance.
Direct-Connect EKG Cable for models:
SE-PC-AHA-CLIP
RE-PC-AHA-BAN
In Stock
- 3M
- 3M Health Care (now Solventum)
- Abbott
- Abbott Nutrition
- Abilify (aripiprazole)
- AccuTec Blades
- ADEEVA
- Advanced Sterilization Products
- Advanced Surgi-Pharm Inc.
- Airway Surgical Appliances
- Alcon
- Allergan Pharmaceutical
- Allied Healthcare Products Inc.
- Almedic Ltd
- Ambu
- AMD Ritmed
- American Diagnostic Corporation
- AMG Medical
- Amsino
- ANB
- ANB Canada
- Ansell
- ArjoHuntleigh
- Aspen Surgical
- Axiom® Medical
- B BRAUN MEDICAL INC.
- B. Braun
- Bard
- Baxter
- BD
- Beckman Coulter
- Belpro Medical
- Bemis Health Care
- bioMerieux
- Bionix
- Bird & Cronin
- Bovie Medical
- BSN Medical
- Bunzl Distribution
- Busse Hospital Disposables
- Canadian Custom Packaging
- Cardinal Health
- CardioMed
- CareFusion
- Caromed Inc
- CHS
- Clinton Industries
- Clorox Canada
- Coloplast
- Convatec
- Cook Medical
- CooperSurgical
- Curbell Medical
- Derma Sciences
- Dermacea
- DeRoyal
- Detecto
- Detecto Scale
- Diversey Care
- DJO
- DJO Canada
- Dover
- Drive Medical
- DUKAL
- DWK Life Sciences
- Dynarex
- Eagle Labs
- Ecolab
- EKO
- Eko Health
- embecta
- ESBE Scientific
- Essity
- Ethicon
- Exactech
- Exciton Technologies Inc
- Exergen Corporation
- FERNO
- FERNO Canada
- Ferris Mfg Corp
- First Healthcare Products
- First Wave® Products Group
- Formedica
- Gate Rehab Development
- Gentell Inc
- Gentell Inc (Derma Sciences)
- Geri Care
- Germiphene
- good sense
- Graham Medical
- Graham-Field
- Harloff
- Health o meter
- Healthline Medical Products
- Hikma
- Hollister
- Hygie
- Hygie Canada
- ICU Medical Canada
- ICU Medical Canada Inc.
- Inc.
- Innovatek Medical Inc
- INOPRO
- Integra LifeSciences
- Invacare Canada
- Invacare Continuing Care
- IPSEN
- Johnson & Johnson
- Kimberly-Clark Professional
- Kohl & Frisch
- Laerdal
- Lassonde Industries Inc.
- Lernapharm
- LIFEPAK
- LILLY
- LLC
- Ltd
- Major
- Manrex Limited
- Marlatek Inc.
- Masimo
- MECP
- Medegen Medical Products
- Medical Indicators
- Medical Indicators (MII)
- Medicom
- MediSure
- Medline
- MEDTRONIC
- Metrex
- Metro
- Midmark
- MJM
- MMSI
- Molnlycke Health Care
- MONICA VIP HA FILLER
- Mylife Diabetescare
- Natus
- NewTech - Vaportek
- Nissha Medical Technologies
- Novo Nordisk
- O-Two Medical Technologies
- O&M Halyard Canada ULC
- Omron Healthcare
- Otsuka Pharmaceutical Co.
- Parsons ADL Inc
- Patterson Medical
- PDC
- PDI
- Pedigo Products
- Performance Health
- PharmaSystems Inc
- PHC Corporation of North America
- Polar Ware
- Posey
- Posey Company
- PPI
- Presource
- Prevent Products
- PTS Diagnostics
- purell
- Puritan Medical Products
- QHP
- Rane Bathing Systems
- Rapid Aid
- Reckitt Benckiser Inc
- Rescue 7
- Revance
- Revanesse® Kiss™
- Riester
- Roche Diagnostics
- Roxon medi-tech ltd
- Safecross First Aid Ltd.
- Sage Products
- Sarstedt
- seca
- SEGUFIX-System
- Sheffmed
- Simport® Scientific
- Smith & Nephew
- Smiths Medical
- Sol-Millenium
- Solic Medical Equipment
- Solventum (formally 3M Health Care)
- Southmedic
- Spacelabs Healthcare
- Span Medical Products Canada
- Span-America Medical Systems
- Spenco
- Stadco Polyproducts
- Starplex
- Starplex Scientific Inc.
- STERIS Healthcare
- Stryker Canada
- Sunmed Group
- SUNMED GROUP PHOENIX
- Supplier (TBD)
- Suretex
- Surgical Specialties Corporation
- Swish
- Symmetry Surgical
- Teleflex
- Teligent
- Terumo
- Thayer Medical
- Thinklabs
- TIDI
- Tollos
- Trudell Medical
- Tuttnauer
- Tytex
- UAL
- UTERMOHLEN
- Venoscope
- Vernacare
- Vinyl Products Limited
- Viscot Medical
- vivacy paris
- VYAIRE
- W. A. Baum Co. Inc.
- Walgreen Health Solution
- WASIP Ltd
- Wealy
- Weaver and Company
- Welch Allyn
- Westech Health Care Ltd.
- Xeomin Filler Treatment
- ZOLL
- Accessories
- adaptor
- Bd
- blood pressure
- Cardiology
- Cardiology - ECG
- Christmas Collection
- Cold Supply Chain
- Collagen Stimulators
- Cotton Masks
- Daily Living Aids
- Dental
- Dermal Fillers
- Diabetic
- Disinfection and Sterilization
- exam gloves
- Face Shield
- finger cot latex
- Food Safety
- Furniture
- hammer
- Harm Reduction
- Hospital Ware
- Infection Prevention and Control
- Instruments and Equipments
- Bathing Safety
- Carts and Storage
- Emergency Kits
- Exam and Diagnostic Equipment
- Lab Equipment
- Beakers
- Blood Collection
- Cassettes
- Centrifuge Supplies
- Chemicals Reagents Solutions and Stains
- Clinical Chemistry Calibrators
- Clinical sample collection/preparation
- Fridges and Freezers
- Funnels
- Inoculation Loops
- Lab Filtration
- Lab Thermometers
- Microscopes Slides and Accessories
- Pipettes
- Scales and Weights
- Specimen Transport
- Test Tubes and Accessories
- Testing Containers
- Testing Kits
- Tools
- Transfer Pipettes
- Mobility Aids and Patient Transport
- Nursing Equipment
- OR Equipment
- Surgical Instruments
- Biopsies
- Bite Blocks
- Calipers and Measuring Instruments
- Cast Cutters
- Clamps & Forceps
- Curettes
- Dilators
- Ear Nose and Throat (ENT) Instruments
- Elevators
- Endoscopic Instruments
- Extractors
- Hammers and Mallets
- Instrument Holders
- Knives
- larynx
- Ligation Instruments
- Nail Nippers and Splitters
- Orthopedic Instruments
- Pin and Wire Cutters
- Probes
- Punches
- Rasps
- Retractors
- Ring Cutters
- Scissors
- Shunts
- Spreaders
- Stapling and Staple Removers
- Surgical Standard Packs
- throat
- Tourniquets & Occluders
- Surgical Supplies
- Needles And Syringes
- Nursing Supplies and Patient Care
- Patient lift
- Pharmaceutical
- PPE
- Prescription
- printer thermal tape
- Replacement Filter
- Respiratory and Anesthesia
- Skin and Wound Care
- Surgical
- Surgical Laser
- Test Kit
- Youth Masks